Master the Art of Taking Your Formula from Concept to Market in Record Time
The Shocking Reality
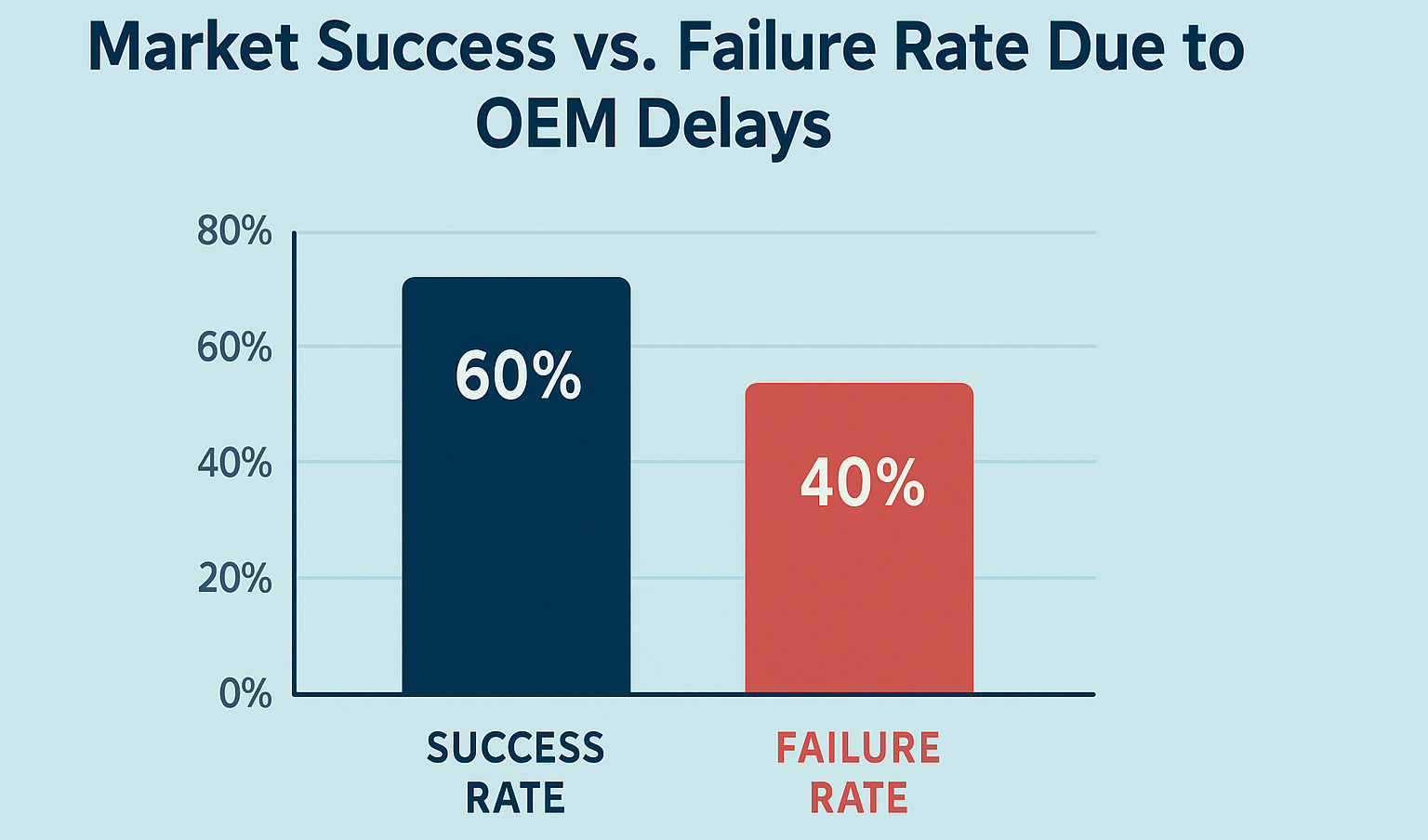
73% of new health supplement brands fail to reach market due to OEM delays. While some competitors launch in just 3 months, many founders find themselves stuck for over a year with no progress. Don’t let your brand become another statistic.
🚀 The global nutraceutical market is growing at 12% annually, but speed to market determines who captures this massive opportunity.
The Four Critical Stages of OEM Success
1. Formulation Design & Compliance (1-3 Months)
Transform your “desired effects” into production-ready formulations that comply with global regulations. This stage can make or break your timeline.

Real-World Example
An Australian sleep aid brand initially wanted GABA + melatonin + magnesium. Since GABA requires additional EU approval, switching to magnesium + melatonin saved 45 days of regulatory waiting.
Pro Tip: Ingredient Whitelisting
Always verify ingredient permissions across target markets. What’s legal in the US might be banned in the EU, and vice versa. This single step can prevent months of delays.
2. Raw Material Procurement & Quality Control (1-2.5 Months)
Source premium ingredients globally while implementing rigorous quality controls. The world’s best curcumin comes from India, probiotics from Denmark – but quality verification is everything.
🔍 Supplier Audit Verify: COA accuracy with third-party testing
Quality Control Disaster Averted
A Vietnamese piperine supplier’s COA showed 95% content, but third-party testing revealed only 82%. This discovery prevented an entire batch from reaching production – and customers.
3. Production & Packaging (1-1.5 Months)
Choose the right production line and master critical process controls. Each product type requires specific expertise and environmental conditions.

The $50,000 Packaging Mistake
A collagen brand skipped vibration testing requirements. Result: 30% of packages arrived with broken bags, requiring 15 days of rework and missing a major e-commerce promotion window.
4. Regulatory Approval & Market Entry (Up to 12 Months)
Navigate complex regulatory landscapes across different markets. This stage shows the biggest variation in timelines between regions.

USA Market FDA registration: 2-4 months
New ingredients: +6-12 months
European Market EFSA health claims: 14 months average
The Veteran’s Playbook: 3 Time-Saving Strategies
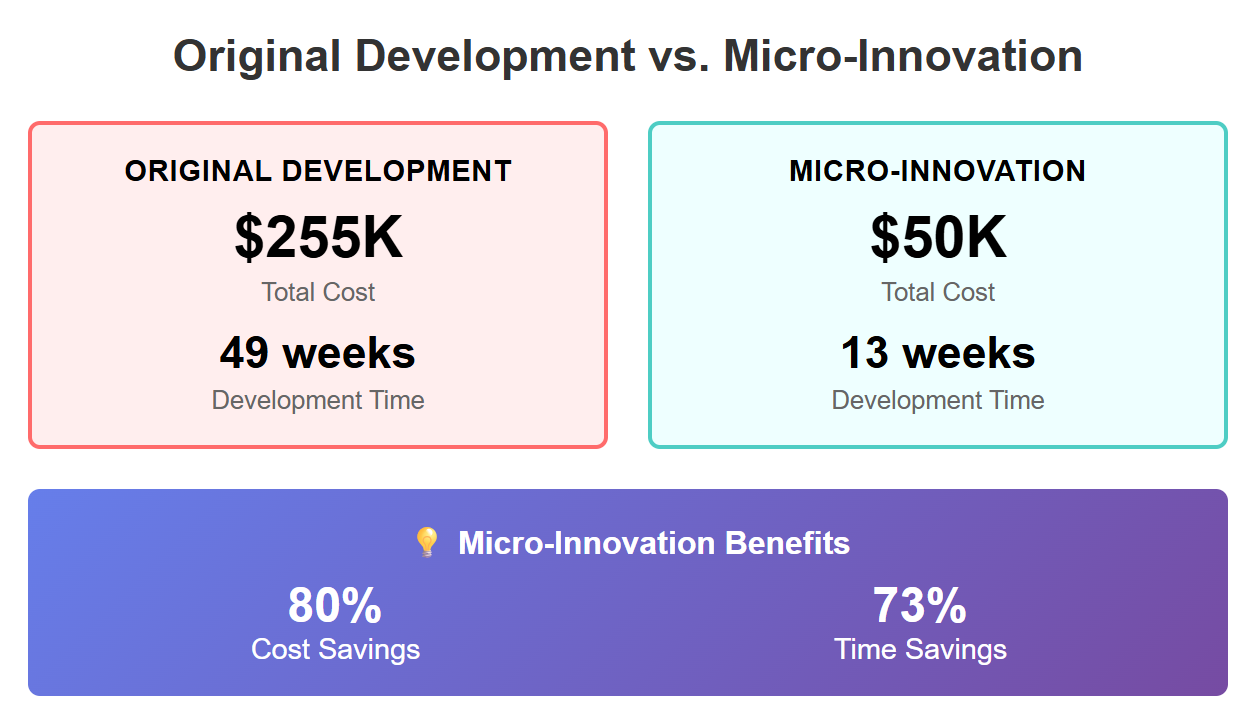
Strategy #1: Formula Micro-Innovation
Abandon the “original obsession.” Fine-tuning proven formulas saves 3 months and $100,000+ in R&D costs compared to starting from scratch. Innovation doesn’t always mean reinventing the wheel.
Strategy #2: The Three-Dimensional Factory Assessment
Hardware: Freeze-drying capabilities, automated systems
Software: Electronic batch records, supply chain management
Experience: Recent similar product manufacturing history
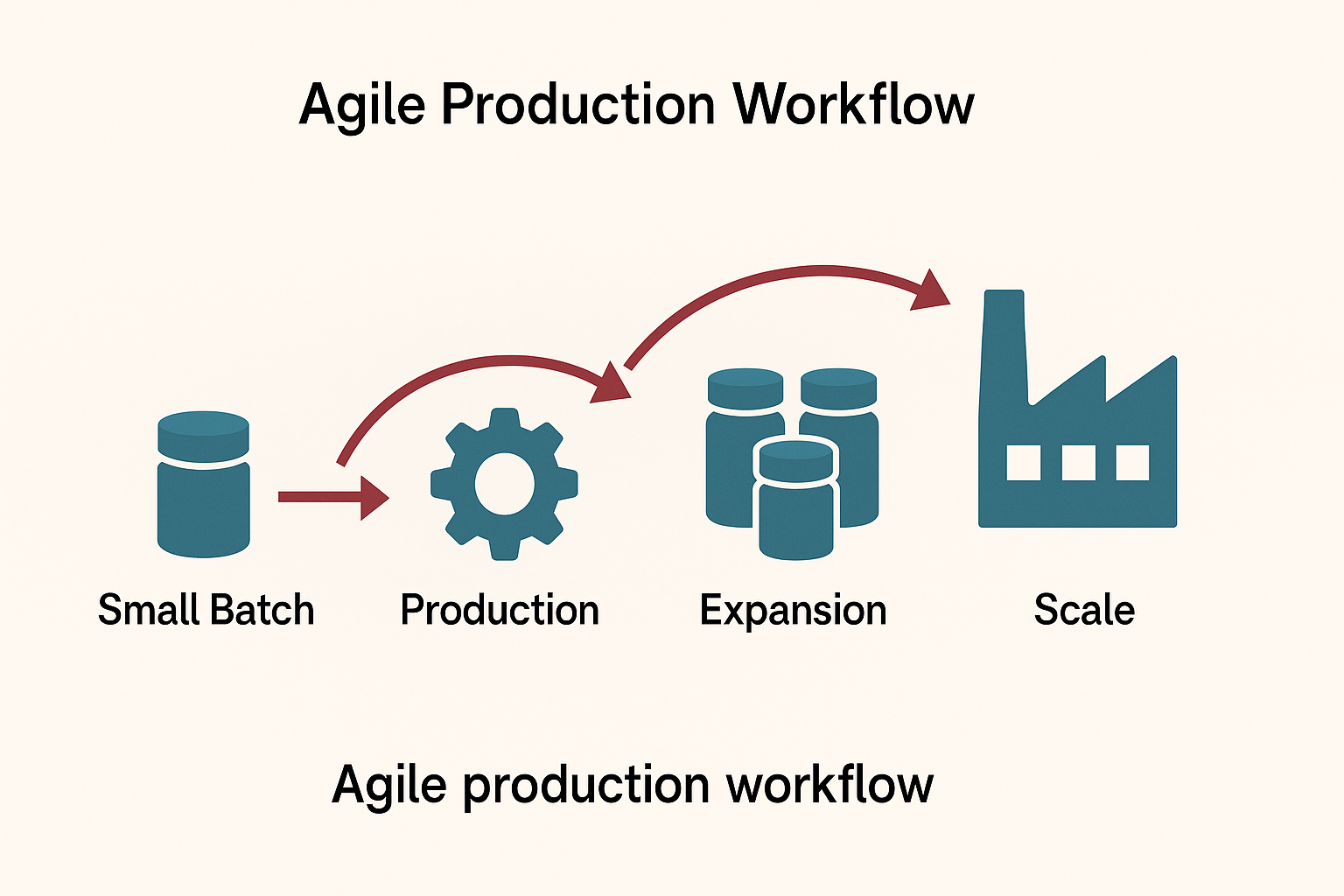
Strategy #3: Agile Small-Batch Production
Start small, scale fast. Test market demand with smaller batches before committing to large-scale production. This approach minimizes risk while maximizing learning opportunities.
Master the Time Code, Win the Market
Health products OEM isn’t just about production execution – it’s a sophisticated orchestration of compliance, supply chain management, and process optimization. In this “fast fish eat slow fish” era, time equals market share, and efficiency determines brand survival. The brands that master this time code will capture the first-mover advantage in the competitive nutraceutical landscape.

Visit our website and get it touch
www.yshherb.com
